

NIDA Center for GWAS in Outbred Rats – 9th Annual Retreat
Our lab was thrilled to participate in the NIDA Center for GWAS in Outbred Rats – 9th Annual Retreat held on Friday, October 27, 2023 organized by Dr. Abraham Palmer. The event was a significant gathering of leading researchers in the field, and our team members actively contributed to the discussions and knowledge exchange.


46th Annual RSA Scientific Meeting in Bellevue, Washington
We are thrilled to announce the participation and achievements of our lab members at the 46th Annual RSA (Research Society on Alcoholism) Scientific Meeting held from June 24-28, 2023, in Bellevue, Washington.


Alcoholism and Stress: A Framework for Future Treatment Strategies
We are proud to share highlights from the recent conference titled "Alcoholism and Stress: A Framework for Future Treatment Strategies," which took place in Volterra, Italy, from May 16-19, 2023.

Introducing the Alcohol Biobank: A Revolutionary Resource for Alcohol Use Disorder Research
We are excited to announce that our laboratory has recently secured funding to support our innovative research project focused on the genetic vulnerability to substance abuse and addiction, specifically alcohol use disorder (AUD).

Welcome our new members
Welcome our two new lab members, Kai Zhao and Jamila Piri! We are thrilled to have them on board and look forward to working together to advance our research.

Picnic Farewell Party to Celebrate Caitlin
We celebrated Caitlin Crook, a valued member of our lab team, who is embarking on an exciting new journey to pursue her PhD at the University of Camerino, Italy.

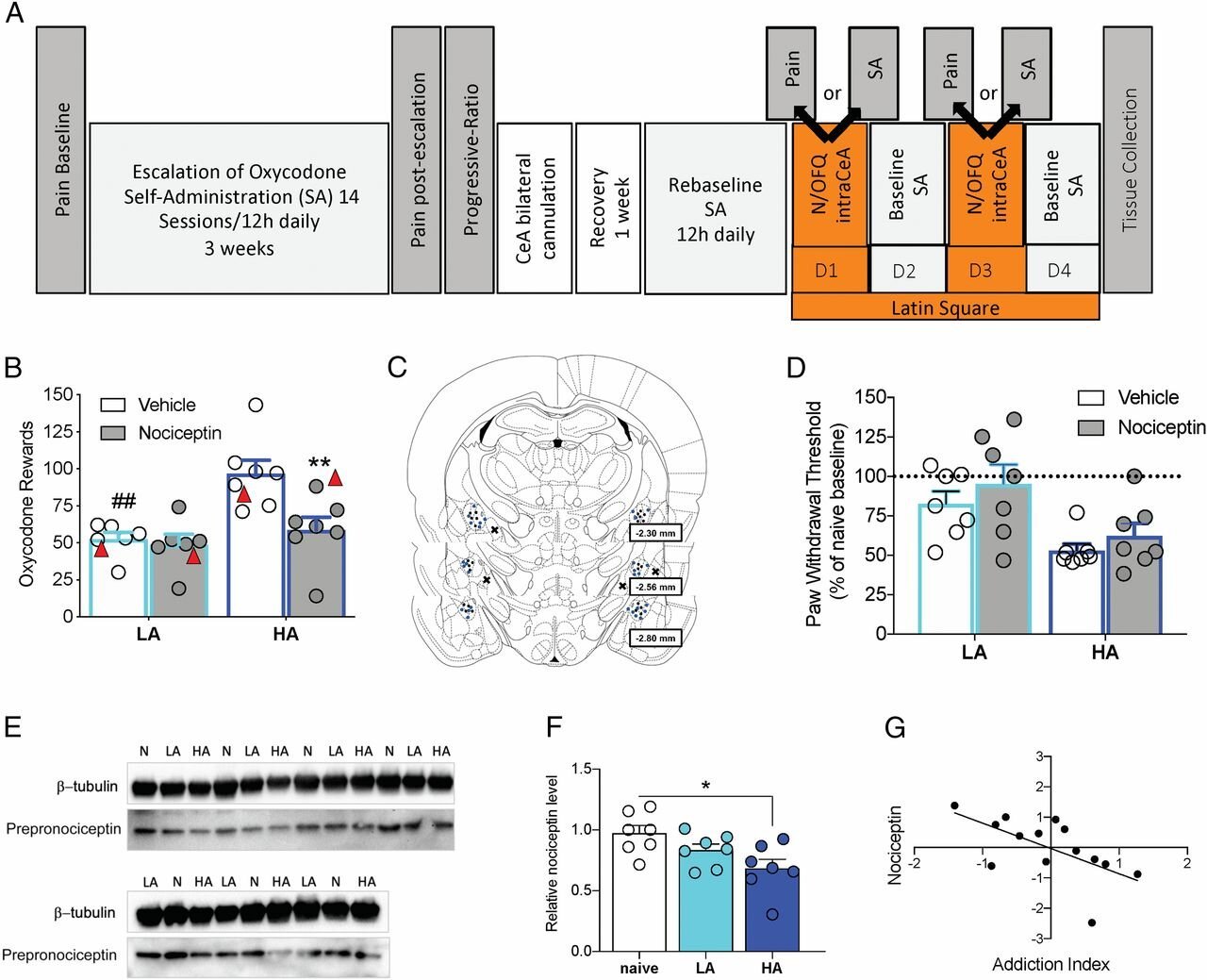
Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA–GABA transmission in highly addicted rats
More than 2 million individuals in the United States currently have a substance use disorder that is related to prescription opioid pain relievers. We identified individual differences in oxycodone addiction-like behaviors in outbred heterogenous stock rats with high and low addiction-like behaviors. We found that the downregulation of nociceptin levels in the central nucleus of the amygdala (CeA) may be responsible for hyper–γ-aminobutyric acid (GABA)ergic tone in the CeA that is observed in individuals who develop addiction-like behaviors. The development of small molecules that target the nociceptin system may have therapeutic efficacy for the treatment of opioid use disorder.
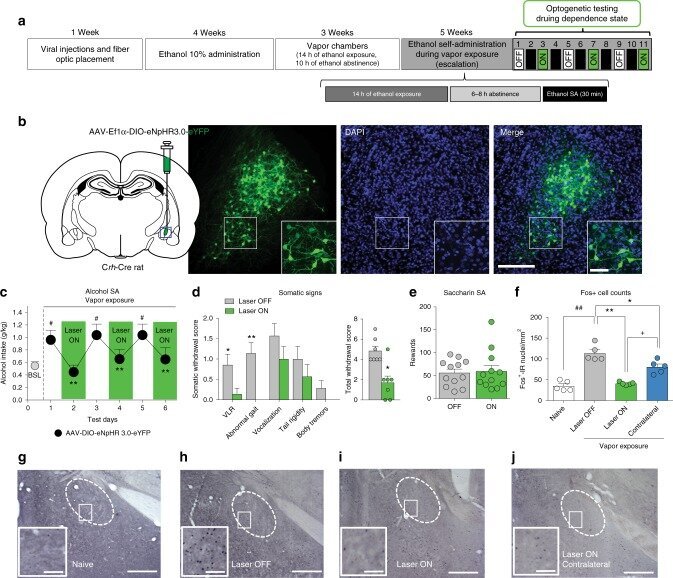
Inactivation of a CRF-dependent amygdalofugal pathway reverses addiction-like behaviors in alcohol-dependent rats.
The activation of a neuronal ensemble in the central nucleus of the amygdala (CeA) during alcohol withdrawal has been hypothesized to induce high levels of alcohol drinking in dependent rats. In the present study we describe that the CeA neuronal ensemble that is activated by withdrawal from chronic alcohol exposure contains ~80% corticotropin-releasing factor (CRF) neurons and that the optogenetic inactivation of these CeA CRF+ neurons prevents recruitment of the neuronal ensemble, decreases the escalation of alcohol drinking, and decreases the intensity of somatic signs of withdrawal. Optogenetic dissection of the downstream neuronal pathways demonstrates that the reversal of addiction-like behaviors is observed after the inhibition of CeA CRF projections to the bed nucleus of the stria terminalis (BNST) and that inhibition of the CRFCeA-BNST pathway is mediated by inhibition of the CRF-CRF1 system and inhibition of BNST cell firing. These results suggest that the CRFCeA-BNST pathway could be targeted for the treatment of excessive drinking in alcohol use disorder.
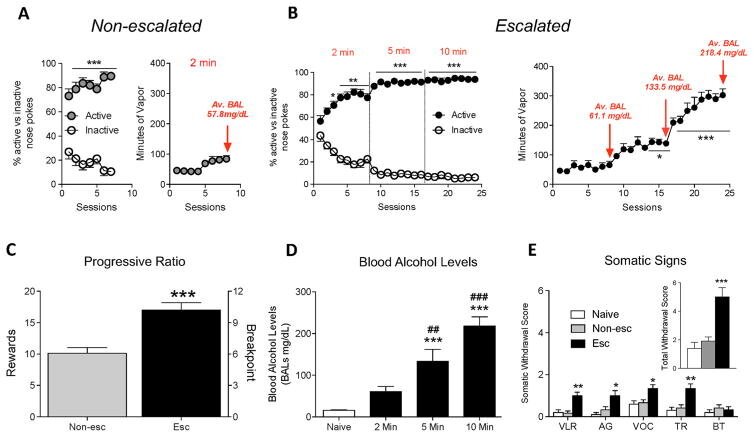
Voluntary induction and maintenance of alcohol dependence in rats using alcohol vapor self-administration.
A major issue in the addiction field is the limited number of animal models of the voluntary induction and maintenance of alcohol dependence in outbred rats. To address this issue, we developed a novel apparatus that vaporizes alcohol for 2-10 min after an active nosepoke response. Male Wistar rats were allowed to self-administer alcohol vapor for 8 h/day every other day for 24 sessions (escalated) or eight sessions (non-escalated). Escalated and non-escalated rats were then tested for progressive ratio responding. Anxiety-like behavior, somatic signs of withdrawal, and hyperalgesia were assessed during acute withdrawal. The results showed that rats exhibited excellent discrimination between the active and inactive operanda (>85%), and the escalated rats quickly increased their blood alcohol levels from ~50 to >200 mg% in ~6 weeks. Compared with non-escalated rats, escalated rats exhibited severe addiction-like behavior, including somatic signs of withdrawal, anxiety-like behavior, hyperalgesia, and higher responding on a progressive ratio schedule of reinforcement. These results demonstrate that outbred rats will voluntarily self-administer alcohol vapor to the point of dependence without the use of forced alcohol administration, sweeteners, food/water restriction, operant pretraining, or behavioral/genetic selection. This novel animal model may be particularly useful for medication development to help unveil the neuronal circuitry that underlies the voluntary induction of alcohol addiction and identify novel molecular targets that are specifically recruited after the voluntary induction and maintenance of alcohol dependence.
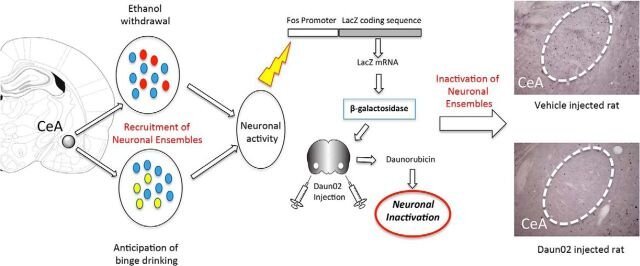
Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence.
Alcohol dependence recruits neurons in the central nucleus of the amygdala (CeA). Here, we found that inactivation of a specific dependence-induced neuronal ensemble in the CeA reversed excessive alcohol drinking and somatic signs of alcohol dependence in rats. These results identify a critical neurobiological mechanism that is required for alcohol dependence, suggesting that targeting dependence neuronal ensembles may lead to a better understanding of the etiology of alcohol use disorders, with implications for diagnosis, prevention, and treatment.
